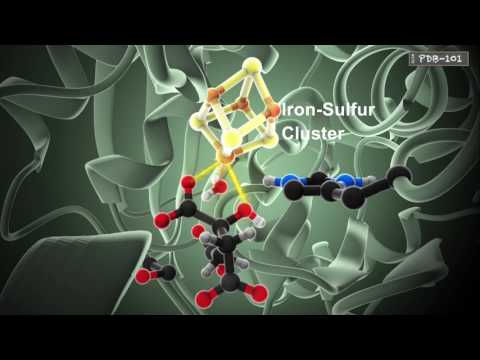
酵素の働き (How Enzymes Work)
Szu-Pei Wu が 2021 年 01 月 14 日 に投稿  この条件に一致する単語はありません
この条件に一致する単語はありませんUS /ɪˈsɛnʃəl/
・
UK /ɪ'senʃl/
US /ˈrek.əɡ.naɪz/
・
UK /ˈrek.əɡ.naɪz/
- v.t.(~が本当であると)認める : 受け入れる;(重要性を)認める;法的権威を尊重する;公にその人の貢献を称賛する;認識する、認知する
US /ˈtrɪɡɚ/
・
UK /'trɪɡə(r)/
- n.引き金;事を開始する装置;きっかけ;トラウマの引き金;トリガー (電子工学);トリガー (コンピュータ);トリガー (釣り)
- v.t.引き起こす;引き起こす;反射を引き起こす
US /ˈslaɪtli/
・
UK /ˈslaɪtli/
エネルギーを使用
すべての単語を解除
発音・解説・フィルター機能を解除