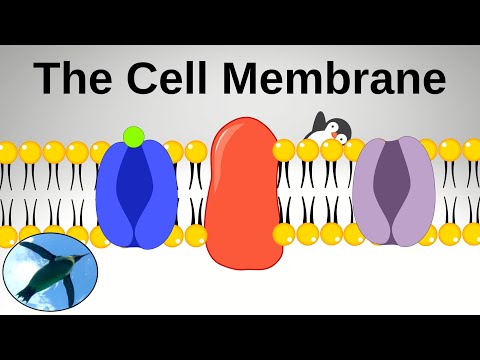
会う...細胞膜だ! (Meet... The Cell Membrane!)
Amy.Lin が 2021 年 01 月 14 日 に投稿  この条件に一致する単語はありません
この条件に一致する単語はありませんUS /ˈstrʌk.tʃɚ/
・
UK /ˈstrʌk.tʃə/
- n. (c./u.)構造;建物
- v.t.組み立てる;組織する
US /ˈbæriɚ/
・
UK /'bærɪə(r)/
- n.障害;バリア;障壁;柵;壁;障害;バリア (コンピューター)
US /ˈmɑlɪˌkjul/
・
UK /ˈmɒlɪkju:l/
- n. (c./u.)形;状況;姿
- v.t.形作る : 方向づける;形をつくる : 形成する
エネルギーを使用
すべての単語を解除
発音・解説・フィルター機能を解除