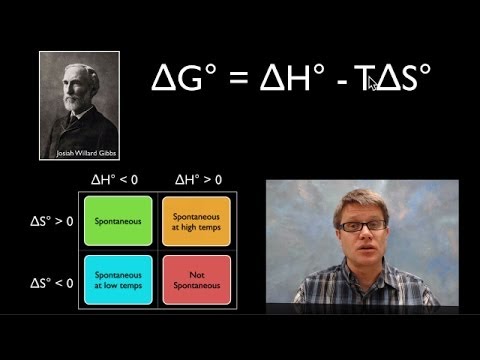
ギブス自由エネルギーの利用 (Using Gibbs Free Energy)
李育真 が 2021 年 01 月 14 日 に投稿  この条件に一致する単語はありません
この条件に一致する単語はありませんUS /ˈprɑsˌɛs, ˈproˌsɛs/
・
UK /prə'ses/
- v.t.(コンピュータの)データを処理する;処理する;処理する;一連の工程を経る;加工する : 加工処理する;理解する
- n. (c./u.)手続き;一連の行為;方法;訴訟手続き;プロセス (コンピューター)
- v.t./i.出場する;計算する;思う;思う
- n.姿 : 体形;数字;人物像;図表;著名人;姿の輪郭;数字
US /ˈnɛɡətɪv/
・
UK /'neɡətɪv/
- n.マイナスの電極;否定文の;「いや」という返事;写真や映画のネガ
- adj.嫌な;負の数の;悲観的な;否定的;陰性の;負の
US /ˈpɑzɪtɪv/
・
UK /ˈpɒzətɪv/
- adj.肯定的な;確実な;電気のプラス極;よい;陽性の;楽観的な;正の;ポジ
- n.ポジ
エネルギーを使用
すべての単語を解除
発音・解説・フィルター機能を解除