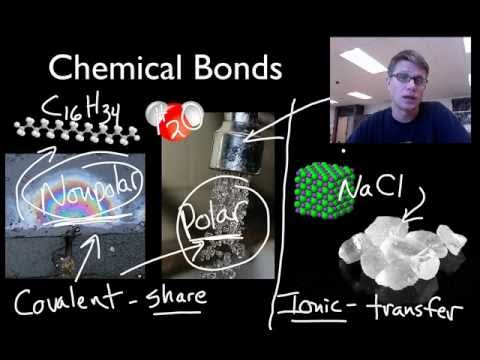
字幕と単語
動画の中の単語
set
US /sɛt/
・
UK /set/
- adj.準備ができている;固定された
- v.t.合わせる;決める;準備する;値付けする;(ある場所 : 時間に)置かれている;設置されている;置く;~な状況に置く;組む;確立する;決意する;作曲する;骨折を整復する
- v.i.固まる;(太陽が)沈む;出発する
- n. (c./u.)一組 : ひとそろい : 一式;(テレビ番組 : 映画の)セット : 撮影現場;(テニスの)セット;(テレビ : ラジオなどの) 受信機;決意
A1 初級TOEIC
もっと見る bond
US /bɑnd/
・
UK /bɒnd/
- n. (c./u.)拘束;絆 : 結びつき;保釈金;借用証書;接着;絆;結合;保証;保税倉庫
- v.t./i.心が触れ合う : 結びつく;くっつける : 接着する
A2 初級TOEIC
もっと見る エネルギーを使用
すべての単語を解除
発音・解説・フィルター機能を解除
