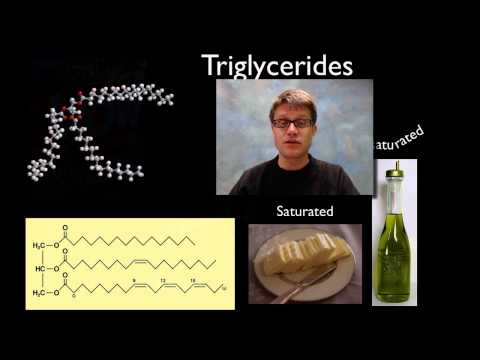
脂質 (Lipids)
Cheng-Hong Liu が 2021 年 01 月 14 日 に投稿  この条件に一致する単語はありません
この条件に一致する単語はありませんUS /ˈbesɪkəli,-kli/
・
UK /ˈbeɪsɪkli/
- v.t./i.(ボールを)ヘディングする;先頭にいる : 最初にいる;向かう;〜の頭である;率いる
- n. (c.)一頭 : 一匹;長 : 代表;コインの表;頭;源
- n. (u.)能力;頭脳 : 知能 : 理性
US /ɪmˈpɔrtnt/
・
UK /ɪmˈpɔ:tnt/
- adj.地位の高い;重要な;重鎮
- n. (u.)重要なこと
- v.t./i.突撃する;請求する;充電する;責任を課す;告訴する
- n. (c./u.)突撃;請求料金;電荷;料金;責任;指示;熱意;告訴
エネルギーを使用
すべての単語を解除
発音・解説・フィルター機能を解除