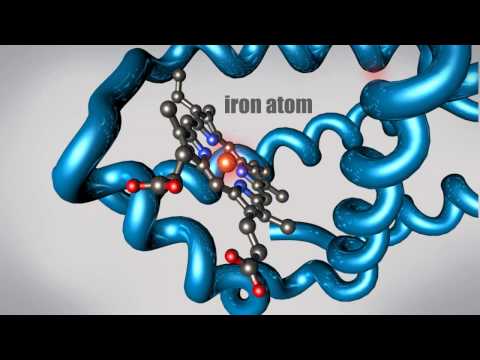
タンパク質とは何か?高分子の立体的な形と機能を知る (What is a Protein? Learn about the 3D shape and function of macromolecules)
Cheng-Hong Liu が 2021 年 01 月 14 日 に投稿  この条件に一致する単語はありません
この条件に一致する単語はありません- n.スポーツのチームの試合記録;記入用紙;形 : 型 : 種類
- v.t.(集団を)結成する : 組織する;形作る : 作り上げる;形成する
US /ˌɪntɚˈækt/
・
UK /ˌɪntər'ækt/
US /ˈprəʊˌtiːn/
・
UK /ˈprəʊti:n/
- v.t./i.突撃する;請求する;充電する;責任を課す;告訴する
- n. (c./u.)突撃;請求料金;電荷;料金;責任;指示;熱意;告訴
エネルギーを使用
すべての単語を解除
発音・解説・フィルター機能を解除