字幕と単語
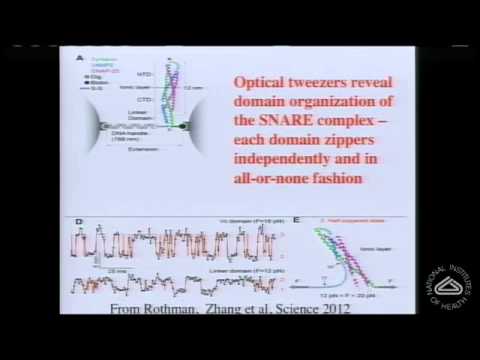
シナプスにおける神経伝達物質の同期放出の分子機構 (The Molecular Mechanism of Synchronous Neurotransmitter Release at Synapses)
00
Precious Annie Liao が 2021 年 01 月 14 日 に投稿保存
動画の中の単語
release
US /rɪ'li:s/
・
UK /rɪ'li:s/
- v.t.自由;発表する、公開する;釈放する;手放す;発表する
- n.自由;新商品を紹介すること;解放;釈放;解放装置;権利放棄書;発表;リリース
A2 初級TOEIC
もっと見る エネルギーを使用
すべての単語を解除
発音・解説・フィルター機能を解除
