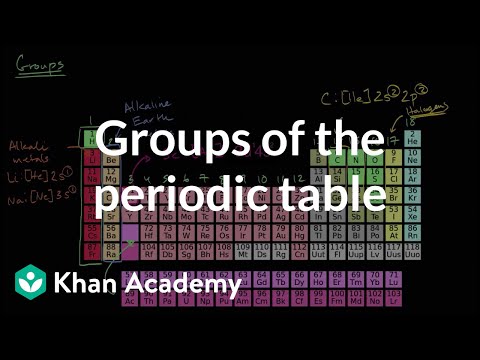
周期表の群|周期表|化学|カーンアカデミー (Groups of the periodic table | Periodic table | Chemistry | Khan Academy)
林宜悉 が 2021 年 01 月 14 日 に投稿  この条件に一致する単語はありません
この条件に一致する単語はありませんUS /ˈprɑːpərli/
・
UK /ˈprɔpəlɪ/
US /ˈslaɪtli/
・
UK /ˈslaɪtli/
- v.i.(ある方向へ)徐々に進む : 向かう
- v.t.世話をする : 面倒を見る
- v.t./i.~する傾向がある
US /ˈkærəktɚ/
・
UK /'kærəktə(r)/
- n.(物語 : 映画 : 演劇などの)登場人物;文字;性格 : 性質;変わっている人;評判
エネルギーを使用
すべての単語を解除
発音・解説・フィルター機能を解除